The research group Functional Materials works in the field of solid-state chemistry with a focus on materials for energy storage and conversion. These can be, for example, intermetallic phases, metal hydrides, nitrides, silicates, chalcogenides or halides. Many projects are interdisciplinary and overlap with crystallography, solid-state physics and materials science.
The synthesis of solids has many facets. The methods range from those using “brute force”, e.g. high temperature and high pressure, to “soft chemistry”, chimie douce, near ambient conditions. They use a variety of techniques, including solid-solid or solid-gas reactions, chemical transport reactions, solvothermal and precursor methods, topotactic reactions, melting reactions, self-sustaining reactions and precipitation from solution - to name but a few. Most of these techniques are used to search exploratively for new substances which then have a potential use in applied research. The planning of targeted syntheses is hampered by the limited knowledge of reaction processes. In situ studies allow this gap to be closed and reaction pathways to be elucidated, which are invaluable for synthesis planning and optimization (see scheme).
Characterization is mainly carried out using in situ X-ray and neutron diffraction, thermal analysis and Raman spectroscopy. The research group Functional Materials deals with a wide range of functional materials and technical processes. The following articles are intended to provide an insight into current research focuses:
Solid-gas reactions play an important role in many technologically important processes such as the smelting of ores, heterogeneous catalysis, the synthesis of solids, hydrogen storage and the corrosion of metals and alloys. Therefore, these reactions are key for the production, application and utilization of many functional materials. Research into such reactions is technically complex due to the often harsh conditions. Neutron diffraction is ideal for characterizing such reactions, as common gases such as H2, N2, O2, H2O, CO oder CO2 contain light elements. These elements are often difficult or impossible to detect in the crystal structure using X-ray diffraction. In addition, the bulky experimental apparatus for in situ characterization can be penetrated more easily by neutrons, as the absorption coefficient of most elements is much lower than that of X-rays. Furthermore, neutrons can be used to investigate not only the crystal structure, but also diffusion, magnetism and lattice vibrations, among other things. For time-resolved investigations of solid-gas reactions, we have constructed a gas pressure cell for elastic neutron diffraction (see image). Furthermore, in situ X-ray diffraction, Raman spectroscopy and thermal analysis, each as a function of temperature, gas pressure and gas flow, are important pillars of the time-resolved monitoring of solid-gas reactions. Our research group focuses on reversible hydrogen storage in solids, ore smelting (reduction of metal oxides) and heterogeneous catalysis.
With the correct orientation of this sapphire single-crystal gas-pressure cell as a sample holder, Bragg reflections of the container material can be completely avoided. This results in very high quality neutron diffraction data with a very low background. This allows crystal structures to be determined with high accuracy as a function of gas pressure and temperature in time-resolved investigations, chemical reactions to be tracked, intermediate stages to be identified and solid-state syntheses to be optimized.
Publications on solid-gas reactions
- S. Keilholz, R. Paul, L. Y. Dorsch, H. Kohlmann, In Situ X-ray Diffraction Studies on the Reduction of V2O5 and WO3 by Using Hydrogen, Chem.–Eur. J. 2023, 29, e202203932; doi.org/10.1002/chem.202203932
- A. Götze, S. C. Stevenson, T. C. Hansen, H. Kohlmann, Hydrogen induced order-disorder effects in FePd3, Crystals 2022, 12, 1704; doi.org/10.3390/cryst12121704
- S. Keilholz, H. Kohlmann, H. Uhlenhut, A. Gabke, M. García-Schollenbruch, In situ X-ray diffraction Studies on the Production Process of Molybdenum, Inorg. Chem. 2022, 61, 10126–10132; doi.org/10.1021/acs.inorgchem.2c01226
- R. Finger, N. Kurtzemann, T. C. Hansen, H. Kohlmann, Design and use of a sapphire single-crystal gas-pressure cell for in situ neutron powder diffraction, J. Appl. Crystallogr. 2021, 54, 839–846; doi.org/10.1107/S1600576721002685
- H. Kohlmann, Looking into the Black Box of Solid-State Synthesis, Eur. J. Inorg. Chem. 2019, 4174–4180; doi: doi.org/10.1002/ejic.201900733
SmCo5 is still one of the strongest permanent magnetic materials with one of the highest coercivities. Its high Curie temperature of around 1020 K and the effective magnetic moment of between seven and eight Bohr magnetons per formula unit qualify SmCo5 for use in so-called super magnets, which are used in turbines, compressors, electric motors, guitars, headphones and NMR spectrometers. Although SmCo5 is the textbook example of permanent magnets par excellence, its magnetic structure above room temperature and a presumed transformation into a ferrimagnetic state at 350 K was surprisingly not yet finally clarified. In this case, neutron diffraction, a valuable tool for determining the magnetic structure, has clearly shown that the magnetic moment of samarium at 650 K has a minimum of 0.2 Bohr magnetons, but remains positive and is still aligned parallel to the magnetic moments of cobalt (see figure, small green arrows). More than 50 years after the discovery of this magnetic material, this work shows the crystal and magnetic structures of SmCo5 from the Curie temperature down to 5 K and proves that it is a ferromagnet throughout.
Publications on magnetic materials
- A. Götze, S. C. Stevenson, T. C. Hansen, H. Kohlmann, Hydrogen induced order-disorder effects in FePd3, Crystals 2022, 12, 1704; doi.org/10.3390/cryst12121704
- H. Kohlmann, T. C. Hansen, V. Nassif, Magnetic Structure of SmCo5 from 5 K to the Curie Temperature, Inorg. Chem. 2018, 57, 1702-1704, DOI: 10.1021/acs.inorgchem.7b02981
Luminescent materials (so-called phosphors) are omnipresent in everyday life --- whether in screens of electronic devices, as light-emitting material in an LED or as fluorescent dye on banknotes. Such photoluminescent materials usually consist of a host lattice into which small amounts of activators are doped. In contrast to most other metal ions, the electronic structure of the activator ion Eu2+ allows the emission wavelength to be influenced by selecting a suitable host structure. The excited state (4f65d1) is influenced by ligand field splitting and even more by a shift of the center of gravity of the d-states through covalent interactions with ligands (nephelauxetic effect). For halides, this effect is very small and the ligand field is weak, leading to a high-energy excited state and the emission of short wavelengths (violet-blue). For hydride ions, on the other hand, the nephelauxetic effect is strong and the center of gravity of the excited state is therefore significantly lower, leading to higher emission wavelengths. The gradual replacement of fluoride ions by hydride ions allows the emission wavelength to be adjusted continuously. However, the largely ionic metal hydrides and hydride fluorides are very sensitive to air, which limits their applications.
Publications on luminescent materials
- C. Pflug, H. Kohlmann, Structural Distortion on Perovskite Type KCaH3–xFx (0.54 ≤ x ≤ 3), Z. Anorg. Allg. Chem. 2020, 646, 175–179
- F. Gehlhaar, R. Finger, N. Zapp, M. Bertmer, H. Kohlmann, LiSr2SiO4H, an Air-Stable Hydride as Host for Eu(II) Luminescence, Inorg. Chem. 2018, 57, 11851−11854, DOI:10.1021/acs.inorgchem.8b01780
- N. Kunkel, A. Meijerink, H. Kohlmann, Bright yellow and green Eu(II) luminescence and vibronic fine structures in LiSrH3, LiBaH3 and their corresponding deuterides, Phys. Chem. Chem. Phys. 2014, 16, 4807-4813, DOI:10.1039/C3CP55102D
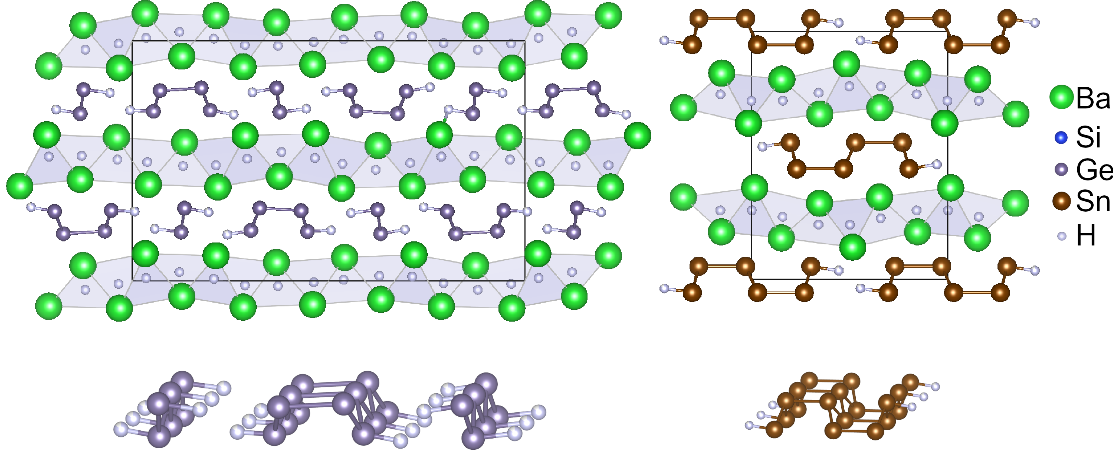
Zintl phases are polar intermetallic compounds at the boundary between ionic salts and metals (M), which consist of a metal from the first or second main group and an element from the thirteenth to sixteenth main group (X). They contain polyanions, which can be explained by the general 8-N rule, assuming an electron transfer from M to X atoms. Extremely exciting observations can be made in the reaction of Zintl phases with hydrogen. A filigree redox chemistry allows the insertion of hydrogen atoms into interstitial sites, the oxidation of polyanions, the formation of new X--X or X--H bonds and the insertion of hydrogen atoms into the X--X bond (see figure). The hydrogenation of Zintl phases produces hydrides with a wide variety of structures and bonding patterns. Systems with light and readily available elements such as CaSi -- Ca3Si3H4 or KSi -- KSiH3 are also interesting hydrogen storage materials.
Publications on Zintl phase hydrides
- A. Werwein, H. Kohlmann, Synthesis and Crystal Structure of BaLaSi2H0.80, Z. Anorg. Allg. Chem. 2020, 646, 1227–1230; doi:10.1002/zaac.202000152
- H. Auer, F. Yang, H. Y. Playford, T. C. Hansen, A. Franz, H. Kohlmann, Covalent Si–H Bonds in the Zintl Phase Hydride CaSiH1+x (x ≤ 1/3), Inorganics 2019, 7, 106; doi:10.3390/inorganics7090106
- A. Werwein, H. Auer, L. Kuske, H. Kohlmann, From Metallic LnTt (Ln = La, Nd; Tt = Si, Ge, Sn) to Electron-precise Zintl Phase Hydrides LnTtH, Z. Anorg. Allg. Chem. 2018, 644, 1532–1539, DOI: 10.1002/zaac.201800062