The group is very active in the field of surface science, which contains topics relevant to heterogeneous catalysis, materials science and interactions at interfaces. Main techniques used to address such questions are photoelectron spectroscopy, x-ray absorption spectroscopy and other surface sensitive methods.
Research projects
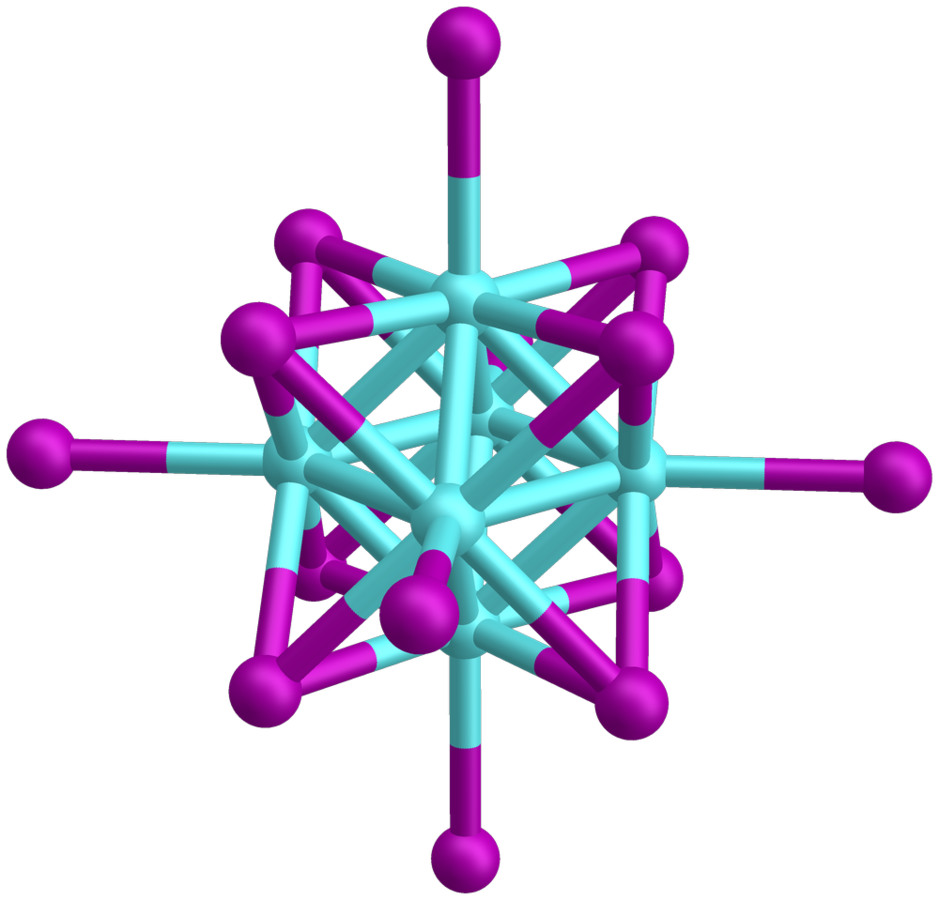
Molybdenum compounds of the form Mo6X142− (X = Cl, Br, I) have interesting photoactive properties due to their particularly long-lived electronic states excited by visible light. For example, these compounds have gained considerable attention as singlet oxygen sensitizers and photofunctional materials.
We build molecular layers of these compounds, obtained from Kaplan Kirakci (Czech Academy of Sciences), on surfaces using different methods. Among other things, we use ion soft landing in cooperation with NFG Warneke, which enables the mass-selective separation of these ionic compounds and their fragments from the gas phase. The produced surfaces are then characterized using surface-sensitive methods such as XPS, IR spectroscopy and LESA-MS and examined for photocatalytic and photosensitive properties.
Oxygen electrocatalysis has attracted considerable attention due to its critical role in the performance of important electrochemical energy conversion devices and energy storage devices such as fuel cells, electrolyzers, and metal-air batteries. Perovskites with the formula A2BO4 have been shown to be promising electrocatalysts for oxygen electrocatalysis due to their appropriate mixed ionic and electronic conductivities. We investigate surface structure and composition as parameters to understand and tune the oxygen electrocatalysis activity of these oxides. We use quantum chemical calculations in combination with detailed characterization, controlled synthesis and testing as a powerful means to develop the fundamental molecular-level knowledge required for the development of efficient non-stoichiometric mixed metal oxides for oxygen electrocatalysis.
X-ray photoelectron spectroscopy is an important and widely used analytical method for determining the chemical composition of samples. X-rays are used to emit photoelectrons that have element-specific kinetic energies. In addition, conclusions about the chemical environment (e.g. binding partner) can be drawn based on the chemical shift. This enables a very specific qualitative analysis. In addition, a quantitative analysis is also possible to determine the proportions of the individual species.
Since the mean free path of electrons is too short at normal pressure, the measurements are usually carried out in ultra-high vacuum. This creates the problem that liquid samples normally cannot be measured. Near ambient pressure XPS (NAPXPS) is a method that attempts to provide a solution to this problem. However, this requires a special device setup, which is rarely available with existing devices.
The approach is therefore to hold the liquids in the meso- and micropores of solids, with the idea of preventing a transition to the gas phase and thus enabling analysis even at very low pressures.
Wavelength Dispersive X-Ray Fluorescence Spectroscopy (WD-XRF) enables the qualitative and quantitative elemental analysis of solid and liquid materials. With the spectrometer at hand, all elements with atom numbers greater than five (from carbon) can be detected. This method is used in cooperation with other institutes such as the Institute for Chemical Technology (industrial glasses, rice husk ash, diatomite and catalysts) or the Institute for Mineralogy, Crystallography and Materials Science (ancient coins).
Equipment
Here a general overview of the machines that are available in the group. More detailed information about current measurements at the science stations can be found at the Research page.
Different methods of sample modification and characterization are available with the spectrometer VG ESCALAB 220i-XL.
- XPS (X-ray photoelectron spectroscopy)
- UPS (Ultraviolet photoelectron spectroscopy)
- LEED (Low energy electron spectroscopy)
- AES (Auger electron spectroscopy)
- SEM (Secondary electron microscopy)
- PLD (Pulsed laser deposition)
- EBE (Electron beam evaporation)
Actually, this equipment is employed within the framework of the described research projects and for external service measurements.